NEWS & MEDIA
Shanghai, China, August 04, 2021 --- Today, Shanghai Huaota Biopharmaceutical Co., Ltd. (hereinafter referred to as "Huaota") received the "Approval Notice for Drug Clinical Trial" approved and issued by National Medical Products Administration for the company's injection HB0030 (anti-TIGIT monoclonal antibody).
HB0030 is an anti-TIGIT humanized monoclonal antibody of lgG1, independently developed by Huaota. It can bind to TIGIT with high affinity, thereby blocking the binding to its ligands (such as CD155). The binding of HB0030 to TIGIT on the surface of T cells and NK cells effectively blocks the binding of TIGIT to CD155, then relieves immunosuppression and reactivates the tumor-killing effect of T cells and NK cells.
Preclinical studies have shown that HB0030 has higher affinity and strong in vitro ADCC/CDC activity and demonstrated strong anti-tumor activity in preclinical studies. HB0030 is planned to use in combination with other product of Huaota, such as HOT-1030 (anti-CD137 monoclonal antibody) and HB0025 (anti-PD-L1/VEGF bispecific antibody) to achieve synergistic or additive effect on tumor inhibition.
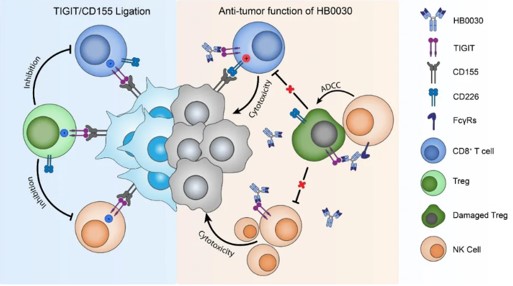
Potential MoA of HB0030
There is currently no anti-TIGIT drugs on the market, and the monoclonal antibody Tiragolumab developed by Roche is in the first class in Phase III clinical trials at present. In January 2021, the combination of Tiragolumab and Tecentriq (anti-PD-L1 monoclonal antibody) for the treatment of NSCLC with high PD-L1 expression was granted by the FDA as a breakthrough therapy. Vibostolimab, developed by Merck, is also currently in a Phase III clinical study in combination with Pembrolizumab (an anti-PD-1 monoclonal antibody) for the treatment of NSCLC. There are different anti-TIGIT antibodies developed by four domestic companies that have launched clinical trials, including BeiGene.
Shanghai Huaota Biopharmaceutical Co., Ltd. is a pharmaceutical company engaged in independent R&D in innovative macromolecule drugs in oncology, autoimmune diseases and fundus lesions. We have established a drug development and pilot test platform for monoclonal antibodies, bispecific antibodies, fusion proteins and ADCs. Huaota is committed to providing high-quality and high-level biological drugs for the global market, meeting the needs of patients for highly available and affordable biological drugs, and practicing the vision of innovation to change the world!