Research and Development
On January 20, 2022, the International Journal of Biological Macromolecules published a research paper titled "Structural and functional insights into a novel pre-clinical-stage antibody targeting IL-17A for treatment of autoimmune diseases". The corresponding author is Dr. Xiangyang Zhu, CEO of Huaota.
HB0017 is a monoclonal antibody targeting interleukin-17 (IL-17) developed by Huaota and intended for the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. As one of the important therapeutic targets, IL-17 is an important pro-inflammatory cytokine that plays a crucial role in the pathological process of various autoimmune diseases including psoriasis, psoriatic arthritis and ankylosing spondylitis.
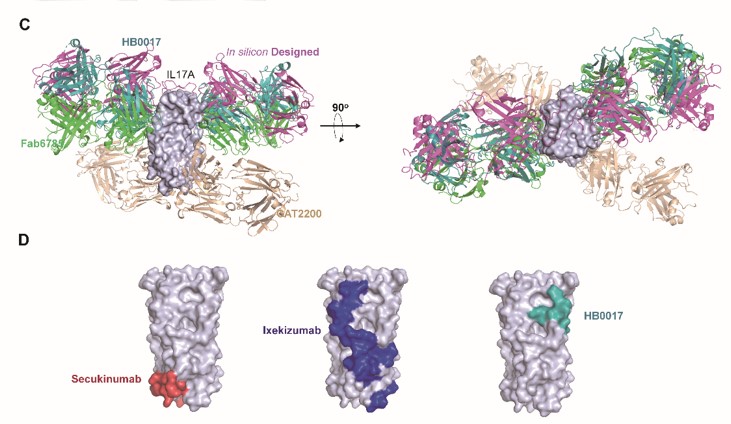
This paper integrates research methods such as structural biology, biochemistry, cell biology and animal models to reveal the unique biological mechanism of HB0017 in the treatment of autoimmune diseases. The crystal structure analysis of HB0017-IL-17A shows that HB0017 has a different binding epitope from the Novartis' commercialized anti-IL-17A antibody secukinumab and Eli Lilly's ixekizumab. This epitope is highly conserved in human, monkey and mouse species with good species crossover properties. Although the contact area between HB0017 and IL-17A is only 500Ǻ2, which is much smaller than 1830 Ǻ2 of secukinumab and 3720 Ǻ2of ixekizumab, the affinity is much stronger than secukinumab, and is equivalent to ixekizumab. Moreover, this interface has great overlap with the binding surface of IL-17A and its receptor IL-17R, so that the binding of IL-17A and its receptor 1L-17R can be blocked very effectively. In addition, pre-clinical results have shown that HB0017 has impressive in-vivo efficacy in mouse psoriasis model, arthritis inflammation model and air sac inflammation model.
Publication link:
https://www.sciencedirect.com/science/article/abs/pii/S0141813022001337
HB0017 is currently in clinical phase 1b. The PASI75 of the participants at a dose of 150 mg reached 100% with potential better than similar antibodies on the market.