NEWS & MEDIA
Shanghai, 17 Dec 2021 – Shanghai Huaota Biopharmaceutical Co., Ltd (“Huaota”) today announced that the U.S. Food and Drug Administration (FDA) has accepted the Investigational New Drug (IND) application of HB0036, a bispecific antibody targeting PD-L1 and TIGIT. HB0036 is a novel bispecific antibody targeting PD-L1/TIGIT. Dr. Xiangyang Zhu, CEO of Huaota, commented on the progress: “HB0036 presents great characteristics in Chemical Manufacturing Control (CMC) process and outstanding antitumor outcome in pre-clinical studies”, said Dr. Zhu. “It has great potential to offer patients a better outcome after treatment, especially those who presents poor response to PD-1/PD-L1 monotherapy. We will launch clinical study as soon as IND is granted.”
Targeting both immune inhibitory receptors, PD-L1 and TIGIT, has been demonstrated a better clinical outcome. Early this month, 10 Dec, Roche released the latest clinical data of combinational use of Atezolizumab (PD-L1 monoclonal antibody) and Tiragolumab (TIGIT monoclonal antibody) as the first-line treatment of people with metastatic non-small cell lung cancer (NSCLC) at European Society for Medical Oncology (ESMO) Congress 2021. The result has shown encouraging efficacy and safety in PD-L1 high-expression population by 71% reduction in the risk of disease worsening or death and a clinically meaningful improvement in overall response rate (ORR) with the combination compared with Atezolizumab alone.
About HB0036
HB0036 is a bispecific antibody designed to bind PD-L1 and TIGIT. It is the second bispecific antibody developed with Huaota Biopharm’s unique multi-function antibody developing platform. Dual blockade of PD-L1/PD-1 and TIGIT/CD155 pathways demonstrated a synergistic anti-tumor effect by overcoming immune suppression of two pathways and enhanceing the activity of T cell and NK cell. Given the HB0036 retains the ADCC function of both anti-PD-L1 and anti-TIGITAbs, it could further kill tumor cells and Treg cells directly, thereby improve the anti-tumor effect.
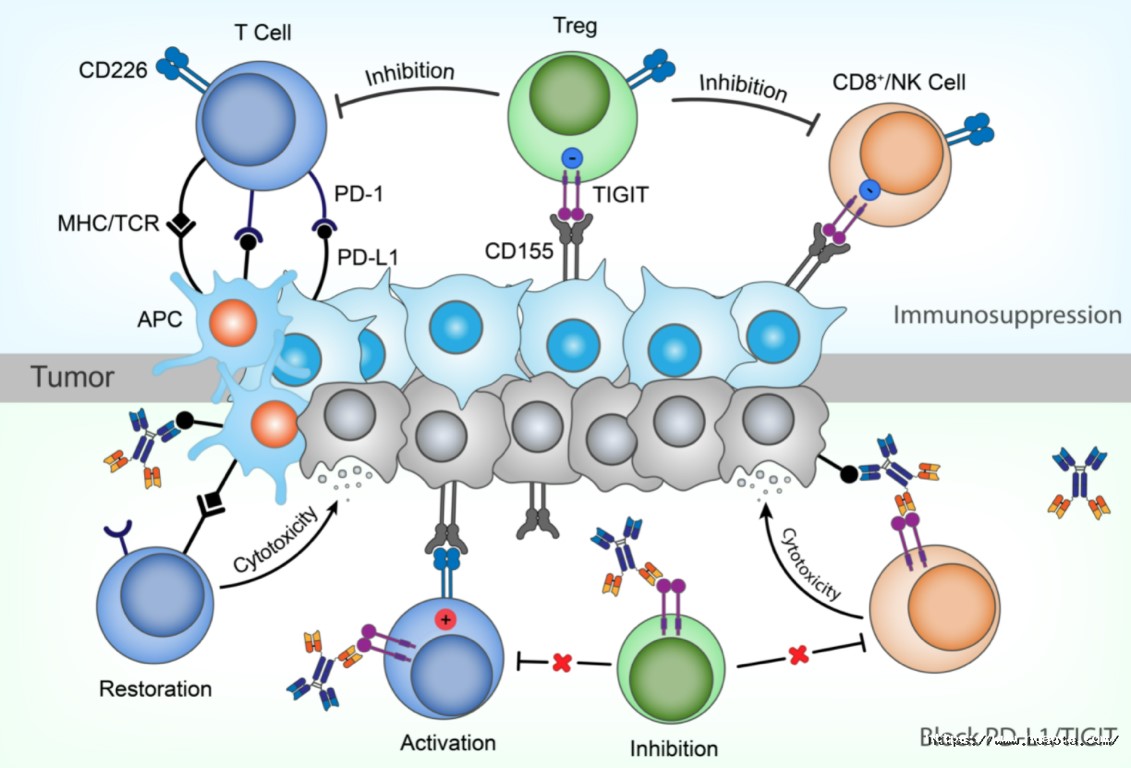
HB0036 shows better efficacy than anti-PD-L1 or anti-TIGIT treatment alone, comparable to the combinational use. Preclinical pharmacokinetic results in non-human primates showed that HB0036 has linear pharmacokinetic characteristics, long half-life and satisfactory pharmacokinetic properties. Toxicological studies had shown that HB0036 has great safety and is well-tolerated in preclinical study. Thus, HB0036 has potential in becoming a new generation of anti-tumor therapy.
Competitive Landscape
At present, there is no PD-L1/TIGIT dual targeting drug reaching market. The leading candidate of its kind is the combinational use of Atezolizumab (anti-PD-L1 monoclonal antibody) and Tiragolumab (anti-TIGIT monoclonal antibody) from Roche. In January 2021, Roche announced thatTiragolumab has been granted Breakthrough Therapy Designation (BTD) by the US Food and Drug Administration (FDA), in combination with Atezolizumab for the first-line treatment of people with NSCLC whose tumours have high PD-L1 expression with no EGFR or ALK genomic tumour aberrations. Today, besides the combination of PD-L1 and TIGIT inhibitor, many companies are also put efforts on developing the possibility bispecific antibody targeting PD-1/TIGIT.